

Question: Calculate the mass percent of chlorine in magnesium chloride.
Chemical Class: A magnesium salt comprising of two chlorine atoms bound to a magnesium atom. Magnesium is highly flammable, especially when powdered or shaved into thin strips, though it is difficult to ignite in mass or bulk. You'll get a detailed solution from a subject matter expert that helps you learn core concepts. Weight of evaporating basin and - Weight of clean evaporating basin. Calculate the mass of magnesium chloride formed. (i)Use the equation to calculate the percentage mass of magnesium in magnesium chloride. The relative atomic mass of magnesium is 24. For instance, if you had a 80.0 g sample of a compound that was 20.0 g element X and 60. (c) The formula of magnesium chloride is MgCl2 The relative formula mass of magnesium chloride is 95. The basic equation mass of element / mass of compound X 100. RAM Mass / Number of moles 0.12 / 0.00592 20.27. Percent composition in chemistry typically refers to the percent each element is of the compound's total mass. Toxicity: ORL-RAT LD50 2800 mg kg-1, ORL-MUS LD50 4700 mg kg-1, IPR-MUS LD50 342 mg kg-1, IMS-MAM LD50 1000 mg kg-1 OU Chemical Safety Data (No longer updated) More details Calculate the relative atomic mass of magnesium. Appearance: white powder OU Chemical Safety Data (No longer updated) More details. #ATOMIC MASS OF MAGNESIUM CHLORIDE HOW TO#
Very soluble in ethanol Kaye & Laby (No longer updated) Explanation of how to find the molar mass of MgCl2 : Magnesium chloride.A few things to consider when finding the molar mass for MgCl2 :- make sure you have. Soluble in water with evolution of heat Alfa Aesar 12315 Soluble in ethanol Kaye & Laby (No longer updated) Completing this calculation gives us a molar mass of magnesium chloride of 95. The molar mass of chlorine is 35.45 grams per mole.
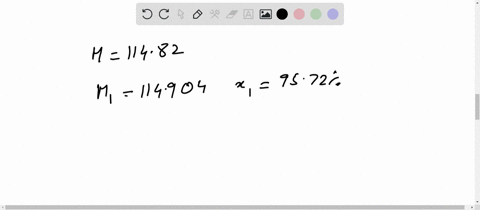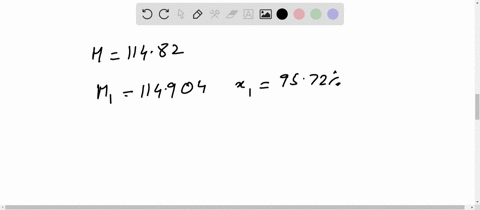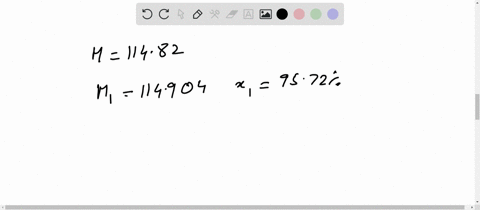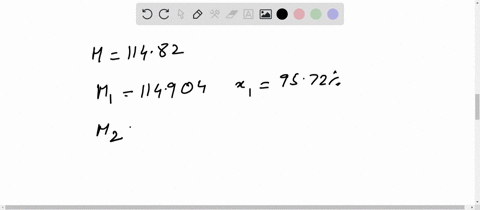
So the molar atomic mass of magnesium is 24.305 grams per mole. Experimental Solubility: 55% w/w in 20?C water Kaye & Laby (No longer updated)ħ3% w/w in 100?C water Kaye & Laby (No longer updated) The relative atomic mass of magnesium is 24.305.Experimental Boiling Point: 1412 ☌ FooDB FDB015357ġ412 ☌ / 760 mmHg Kaye & Laby (No longer updated) Magnesium reacts with hydrochloric acid according to the equation: Mg (s) + 2 HCl (aq) -> MgCl2(aq) + H2(g) This demonstration can be used to illustrate the characteristic reaction of metals with acid, a single replacement reaction, or to demonstrate the generation of hydrogen gas.Experimental Physico-chemical Properties.



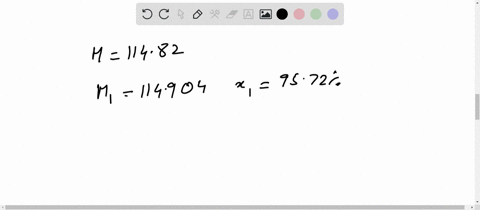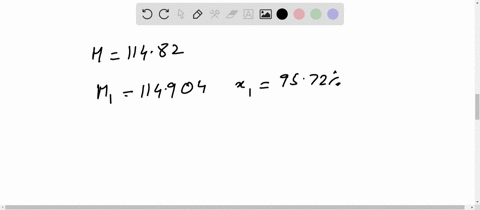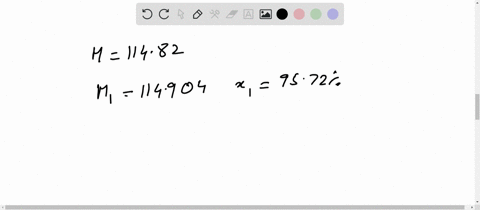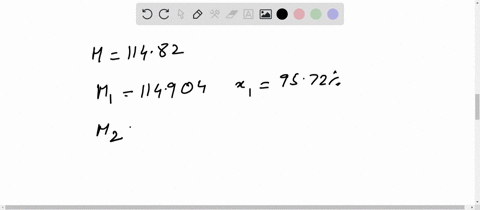


 0 kommentar(er)
0 kommentar(er)
